shelf life calculator for pharmaceutical products
Each batch is distinguished by its own shelf life which can be called the true batch shelf life. This length of time varies depending on the type.
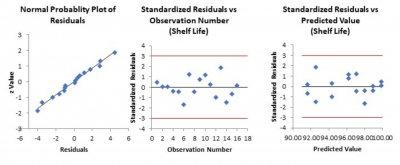
Standardized Residual Definition
Therefore developing and instituting best scientific methods at each step supports the ongoing Quality-by.

. Also it is necessary to find the mark. C Co - k x t C518 - 0267 x t v. A pharmaceutical product is typically manufactured in batches.
Up to 10 cash back Batch and Product Shelf Life. At least three distinct steps are necessary to gain knowledge and understanding of a product or process. For this 125 mL system suitability product shelf life is defensible beyond 360 days twice the stated shelf life of 180 days.
A batch is a fixed quantity of product for example 100000 tablets. For details on the calculation of the shelf life for each case go to the section that describes the calculation when there is only one specification limit. Understand MichaelisMenten nonlinear kinetic behavior and linearization techniques.
The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date. Half life is t12 and shelf life is the time takes to degrade 10. Understand the basis for transition-state theory and its application to chemical kinetics.
Expiry with date in days in months or in years. In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field. The same equation can be used for calculating the shelf life.
A pharmaceutical product is typically manufactured in batches. Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various. After entering data you push button Check and calculator will show you if.
Batch and Product Shelf Life. Let ˆθbe an estimator of the true shelf-lifeθbased on y. 1-Attached is a file describing the calculation method for half life.
With the USP acceptance criteria of 100 15 from USP. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically.
Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data. Similarly if date of manufacture of a drug product is 31st Dec 2016 and drug product has a shelf life of 2 years then the date of expiry shall be Nov 2018. Accelerated aging studies can be used for shelf life determination but must later.
θη αβNotethatαis the average drug characteristic at the time of manufacture iex 0 which is usually larger thanηThusθ0. Shelf life is determined by the evaluation of whole stability data of the product. The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life.
The shelf life of a product may vary between different countriesregions depending on regulatory requirements. SHELF LIFE CALCULATION b N x ƩXY -ƩXx Ʃy -3024 N x ƩX² - ƩX² 11340 b -0267 mgmonth k 0267 a ӯ-b x x a 518 ivThe equationfora straightline bestfitis therefore. Long time studies on pharmaceutical products are carried out over extended time periods till the formulation fails its specifications under the recommended storage conditions.
The quality within each step influences the quality of the resulting knowledge. Establishing the Shelf Life of Pharmaceutical Products. Calculate half-life and shelf life of pharmaceutical products and drugs.
The batch is a single sample of the pharmaceutical products manufacturing process at. Accelerated stability testing and shelf-life calculation. The variance estimateS²xyrepresentsthe variabilityof tabletpotencyatafixedtimeassumingitisequivalentacross all time points.
Shelf life is a product of physical microbiological and chemical processes triggered by any one of a multitude of contributing factors. A batch is a fixed quantity of product for example 100000 tablets. Interpret pHrate profiles and kinetic data.
To calculate the shelf life when the calculations have two limits that are both relevant to the. The shelf life for a pharmaceutical product is taken to be the minimum shelf life for batches on stability. Shelf life calculator for pharmaceutical products.
Depending on the response the confidence interval may be one or two sided. QUANTILE REGRESSION CALIBRATION. For eg If the date of manufacture of a drug product is 5 Dec 2016 and the drug product has a shelf life of 2 years then the date of expiry shall be Nov 2018.
As with the single-use products data presented for bulk CRMs were acquired using bulk products stored at 4C 2C over the course of the study. Typically storage is done at 250C - 20C and RH of 60 - 5 for up to 60 months. I am looking for a shelf life calculator if anyone can help please Basically you input the following info Date of manufacture of product 112012 Date Goods Recd 2732012 Date Goods will Expire 1752014 If the answer is over 75 of shelf life left show result and a pass.
Thus the true shelf-life denoted byθ is the solution ofηαβx hence. This online service helps you to know how long your product is in good condition. The batch is a single sample of the pharmaceutical products manufacturing process at.
This guideline applies to human and veterinary medicines. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration. The shelf life for a pharmaceutical batch is defined as the maximum storage period within which the 95 confidence interval for the batch mean response level remains within the specification range.
A products shelf life generally means the length of time you can expect a product to look and act as expected and to stay safe for use. New Drug Substances and Products presented at the FDA - GPhA Spring workshop in 2012 2013.

An Evidence Based Approach To Nonoperative Management Of Traumatic Hemorrhagic Shock In The Emergency Department Trauma Cme

Compressed Air In Glass Manufacturing Quincy Compressor

How Design Of Experiments Doe Helped A Pharma Company Extend Product Shelf Life

Best Before And Expiry Dates For Food And Drugs Gastrointestinal Society

Aerosol Packaging Archives Packagingguruji

Extension Of Covaxin S Shelf Life Has Been Mishandled

How Design Of Experiments Doe Helped A Pharma Company Extend Product Shelf Life

Manufacturing Date And Expiry Date Of Various Paracetamol Tablets Used Download Table

Calculation Of The Total Dispensing Score Maximum Score 16 Download Table

Novo Nordisk Product Storage Stability Information Novo Nordisk Medical

Accelerated Shelf Life Testing A Basic Understanding Food Marketing Technology

Cross Permeation Simulation Constantia Flexibles

How Design Of Experiments Doe Helped A Pharma Company Extend Product Shelf Life

Plant Based Businesses R D Tax Credits Calculate Your Claim Striketax Com

Accelerated Shelf Life Testing A Basic Understanding Food Marketing Technology

Augmentin Duo 400 57 Powder For Oral Suspension Mixed Fruit Flavour Summary Of Product Characteristics Smpc Emc
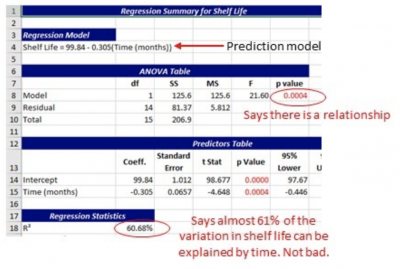
Standardized Residual Definition

Manufacturing Date And Expiry Date Of Various Paracetamol Tablets Used Download Table
